Low sodium salt contains the natural radioactive substance “potassium-40”. How about asking what the “consumers” think?
When we talk about “potassium”, what are we talking about? Is it a banana? Or table salt?
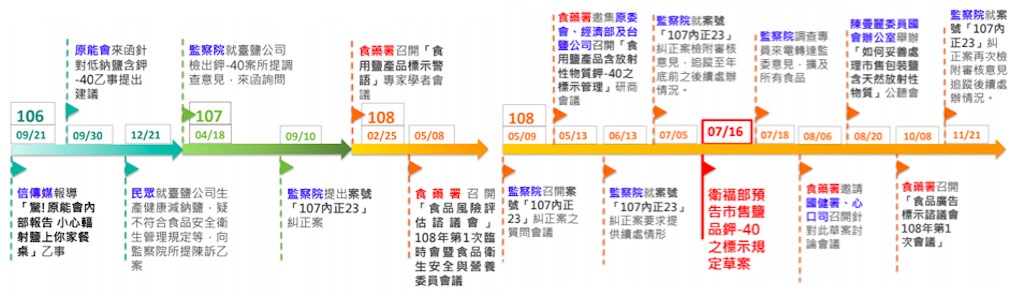
On March 27th, the theme of the 61st collaborative meeting sounded awkward and was difficult to understand. It was about e regulatory notice: “Marking requirements for food additives added to commercially available packaged edible salt containing the naturally radioactive substance potassium-40”.
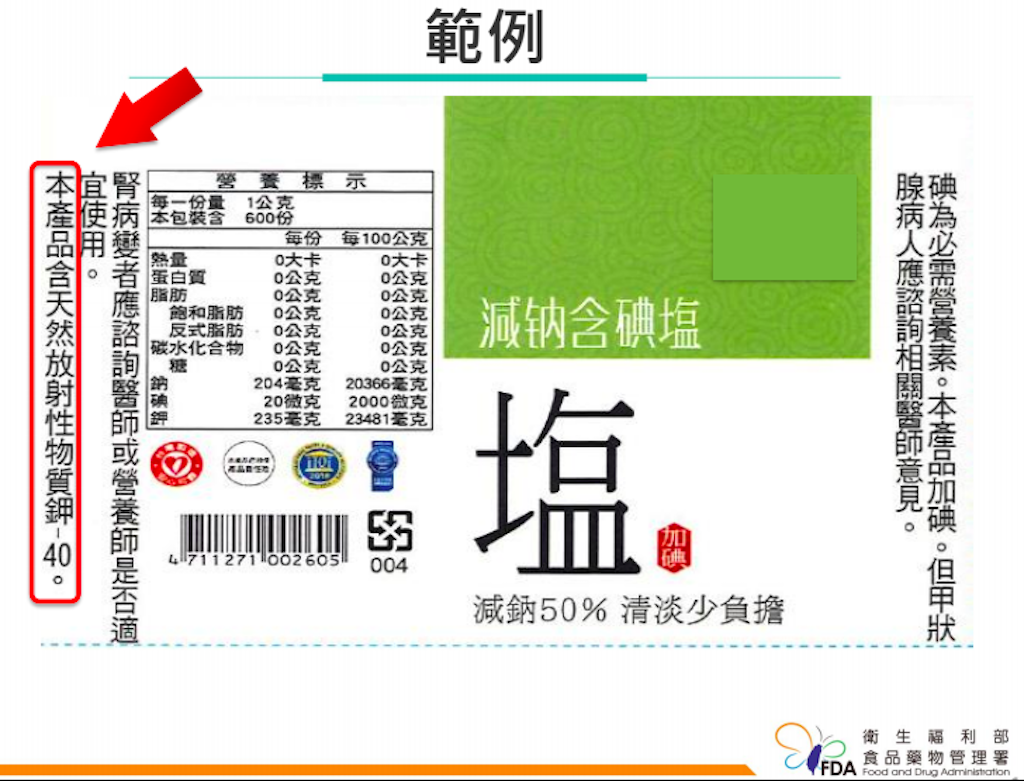
Potassium is a chemical element represented by the symbol “K”. If you add a number “40” after “potassium”, that is, search for “potassium-40” on the Internet as a radioactive isotope of potassium, you will find that what is called “low sodium salt” actually has “potassium chloride” as an alternative component. The question is if the natural radioactivity of “potassium-40” has any effect on the human body and whether it is necessary to mark a warning on the salt packaging.
In September 2018, the Control Yuan made adjustments to several products from Taiyen Biotech, and requested the Ministry of Health and Welfare design labels stating that the product contained naturally radioactive “potassium-40”, and the FDA then posted the draft label on the “crowdtalk” of the National Development Council’s public policy network participation platform.
This discussion on potassium chloride didn’t start after the Control Yuan issued a correction. The Taiwan Food and Drug Administration has faced this issue for a long time: What is a naturally occurring radioactive material? Is low sodium salt containing “potassium-40” really harmful to the human body? What are the good and bad effects of potassium on the human body? People with different perspectives have their own views.
Source: Food and Drug Administration briefing on the day of the collaborative meeting
In previous discussions, some people affirmed the benefits of potassium to the human body from a nutritional point of view, worrying that people will panic when they see the label, so they wouldn’t dare eat low-sodium salts, but it is not beneficial to their health; some people from the perspective of the right of people to know - they think that as long as there is radiation, there are certain risks. We should not assume that consumers would panic. Manufacturers should be regulated to label products with natural emissions; some people believe that the hazards of radiation are ultimately a belief. Or the dissemination of knowledge is also in vain; some people think that countries and international organizations around the world do not think that this situation needs to be a priority. We do not need to be alone in the world, and even have the risk of adverse foreign trade. (For details, see the compiled issue case manual.)
In the 33rd monthly meeting of Participation Officers (PO) last December, the “Provisions on the Marking of Food Additives Containing Natural Radioactive Potassium-40 in Commercially Packaged Edible Salts” was announced in advance by the Food and Drug Administration as the theme of the 61st open government collaborative meeting.
This case was selected online at the monthly meeting, perhaps not only because this topic has a very enthusiastic message discussion on the “join” platform, but also because Taiwanese people are very concerned about food safety issues, and especially because table salt is used almost every day. Therefore, this collaborative meeting was jointly prepared by the Ministry of Health, the Food and Drug Administration and the National Health Administration, the Energy Association, the Ministry of Economic Affairs, and other inter-ministerial meetings.
From “the battle of scientific knowledge” to the shift to “maximum consumer rights” as the core issue
From the perspective of media reports and public opinion, it is not difficult to find that the main contention of this issue lies in “addition of potassium to table salt instead of sodium” as a good starting point for public health, and “natural radioactive substances in table salt must be warned to have “Radiation hazard risk” is also based on the original intention of the public’s health. Although there are completely different opinions on the packaging, the two parties are not the same.
In the first working meeting, we convened colleagues across different ministries and looked forward to how the collaborative meetings could clarify scientific knowledge. Scientific knowledge refers not only to the radiation risks that is the media’s concern, but also to the collection of knowledge in various fields such as food science, nutrition, and trade. We hope that through the design guidance and open and transparent technology that PDIS is good at, people from different positions and different viewpoints can gather together to provide a stepping stone for the discussion of related topics in the future.
However, we also quickly discovered in the process of interviews and data collection by the Ministry of Education that even though scientific knowledge has its rigor and objectivity, people’s perception of risk in different situations may be subjective and affected by various social factors. At the same time, whether the work of popularizing science as a professional can achieve good results in a single collaborative meeting that takes into account the needs of cross-ministerial meetings, without being disturbed by the gap between fields but also how these words are interpreted in the meeting. This variable also makes us think about how to better position the issue.
So, we return to the original intention of the collaborative meeting, which is from the perspective of “seeking common ground while putting differences aside”, so that people with various roles and identities are at least willing to sit together under the basic consensus and listen to each other’s concerns.
As mentioned earlier, all parties take “citizen’s health” as the starting point. So should “citizens” as consumers/users of salt products also play a role in collaborative meetings? At the same time, are all stakeholders involved in this issue, including government departments, experts and scholars, enterprises, and civil society groups, but not people who consume salt products?
Source: FDA briefing on the day of the collaborative meeting
In the follow-up preparation meeting, after many discussions and thoughts, we confirmed the core issue of the collaborative meeting: In response to the draft notice of the Ministry of Health and Welfare, “What kind of labeling method is in the best interests of consumers?”
It must be stated clearly that consumer interests can be achieved in many ways. “Labelling” is a way to do it, and “not labeling” is also a way to do it. The “consumer’s right to know” is very important, but the right to know is only one of the rights and interests. Perhaps there are more rights and interests that we have not yet imagined, which are the parts that consumers care more about.
After redefining the core issues of the collaborative meeting, the organizer also stated that, in addition to the scholars, experts, and group representatives that were involved in the case in the past, they are willing to find multiple people who were “salt consumers” to attend the meeting. At that time, the discussions of each group in the collaborative meeting can ensure that the voice of the “consumer” is heard.
Collaborative meeting with epidemic prevention as the highest guiding principle
An incident was encountered during the preparation of the potassium chloride case, which was the impact of the epidemic. The Ministry of Health and Welfare bears the brunt of the epidemic-related work. The Ministry of Economic Affairs, the co-organizer, must also support related matters such as production and dispatch of epidemic prevention materials, so the collaborative meeting was postponed. As with all physical gathering activities, we have continued to follow the progress of the epidemic during the changing preparation period to ensure that the activities must comply with the guidelines of the Central Epidemic Command Center.
On the day of the event, in addition to the routine amount of temperature testing, hand cleaning and disinfection, we also made adjustments in the venue arrangement to widen the seat spacing of participants, and at the same time ensure that all participants wear masks on-site and there was no eating or drinking. After several considerations, the group discussion meeting format was retained, and the small table leader who led the discussion also communicated first, including how to ensure that the members of the group can communicate with each other within the appropriate range, and how to use the limited arrangement of tables and chairs to guide the discussion. Although the trust established face to face and jointly established in the place is important, it must also be weighed against the risk of infection, and explore the appropriate interaction mode in the context.

Source: PDIS livestream on the day of the collaborative meeting
We always encourage people to participate in the discussion through the Internet, so this case also uses live broadcast technology, with the Sli.do anonymous chat room, and supplemented by Miro to gather opinions from groups and online channels when hosting. With the host controlling the tempo, the question and answer are also conducted more efficiently, reducing the time for gathering, and avoiding the discomfort of everyone wearing a mask indoors for an extended period.
Due to the diverse composition of participants, the leaders of each group need to weigh in the discussion: will the power of discourse brought by knowledge cause some people to feel squeezed during the interaction? The PO and PDIS teams of the Ministry of Economic Affairs, the Ministry of Science and Technology, as the small group leaders to guide the discussion, can participate in the conference composed of heterogeneous participants smoothly in the state of taking into account the multiple objectives due to participating in the preparatory process and understanding the transition of the conference goals.
At the end of the meeting, as the Digital Minister said, “Today, the Food and Drug Administration and related units are so important in the epidemic prevention task. After this meeting, we will open the meeting of the hood 2.0 to hold such a public consultation. It is very sincere to invite interested parties, whether it is to participate on the spot, or a considerable number of friends to watch the live broadcast and participate remotely together. “Even if the epidemic prevention is like a war, the work of open government has not stopped.”
Source: Verbatim transcript on the day of the collaborative meeting
Potassium chloride: An inspiration for designing public consultation procedures
The source of this collaborative meeting came from the “Talk” section of the Join platform, which is the online discussion forum for proposed regulation drafts. Unlike the public proposal stage, because the notice process itself is already the second half of the policy process, even when dealing with physical meetings, the discussion space will usually be narrower and closer to common administrative procedures such as public hearings and briefings. As a result, this meeting is mostly focused on ministry briefings and information clarification, but we are still constantly trying to reset the “core issues” to make the collaborative meeting different from public hearings and expert consultations that have been held in the past. This process highlights the value of open government: “with the people”.
From “posted ideas” as the source, it is easier for us to focus on why than 5,000 people even signed onto this issue? What they care about. From the perspective of “crowdtalk” as the source of the case, our focus is more likely to be on the context of the ministry’s advance notice of regulations? But whether it is a little more demand from the public side or a little more demand from the government side, the “collaborative meeting” is a dialogue platform for the government and the people to understand each other. The core issues often need to be multi-participating, including the main co-sponsoring of the ministry, non-governmental unit stakeholders, and the PDIS team in between.
(Reference: Collaboration meeting: “Core Question Discovery” across sectors)
The debate around low sodium salt is based on the investigation report of the public and the Control Yuan with the “consumer’s right to know” as the initial motivation, allowing the responsible agency to work on the “label” and publish a draft notice, and then communication continued through the online discussion area. The “no labeling” point of view came from multiple positions and practices. When it comes to collaborative meetings, we use the broader “consumer rights and interests” as an entry point of view, so that we are not limited to the “right to know” framework. As we frame this issue, we can also see the traces left by different points of view in the previous discussions.
When open government work emphasizes the orientation of “citizen participation”, it is necessary to measure the status of policies, the nature of issues and various objective conditions, and decide how to plan a consultation process. Sometimes a small number of stakeholders who are heavily influenced by the policy have to play a more important role; sometimes the entrance of the general public can make the consultation process more legitimate; sometimes sampling based on demographic characteristics or based on a more representative consideration of interest groups.
This case, from the seemingly high-threshold discussion of scientific knowledge, was reoriented to professionals in various fields; later it became targetted towards the general public and consumers. Policymakers, professionals, and consumers are now placed in the same field. This process of “re-setting” not only advances the packaging policy of salt products but also adds color to our experience in jointly designing the public consultation process.
 (This work is licensed under a Creative Commons Attribution 4.0 International License.)
(This work is licensed under a Creative Commons Attribution 4.0 International License.)
 En-en Hsu
En-en Hsu